Pediatric storage and handling for VAQTA
Condition on arrival
Should not be frozen. Refrigerate on arrival.1
Storage
Store vaccine at 2°C to 8°C (36°F to 46°F). DO NOT FREEZE since freezing destroys potency.
How supplied
VAQTA is available in prefilled Luer-Lok® syringes.
Pediatric/Adolescent formulations
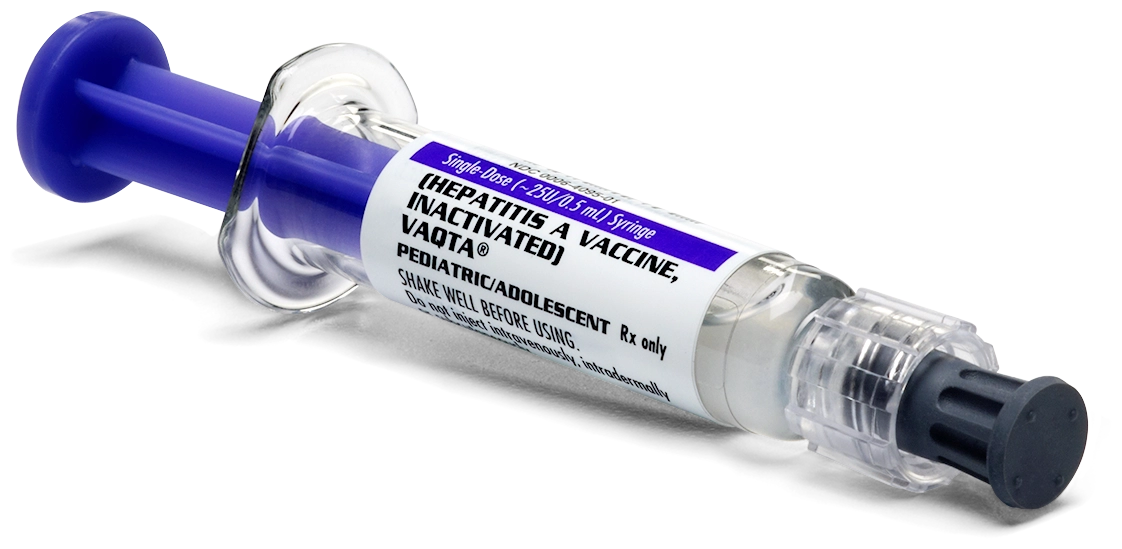
Not shown actual size
NDC 0006-4095-02
Brands mentioned are the trademarks of their respective owners.
General tips
- If you have questions about the condition of the vaccine at the time of delivery, you should immediately place vaccine in recommended storage and call the Merck Vaccine Customer Center at 877.VAX.MERCK (877.829.6372).
- Rotate stock so that doses with the earliest expiration dates are used first.1
- Ensure that the refrigerator is plugged into an outlet in a protected area where it cannot be disconnected accidentally.1
- Vaccines For Children (VFC) Program providers should separate and identify VFC and other vaccines purchased with public funds within the storage unit. In addition, clearly label the space where the vaccine is placed to help staff choose the appropriate vaccine.1
- It is important to use a separate sterile syringe and needle for each individual patient to prevent transmission of infectious agents from one person to another.2
- For general questions concerning the proper storage and handling of Merck vaccines, please contact the Merck Vaccine Customer Helpline at 800.MERCK.90 (800.637.2590), Monday through Friday, 8:00 AM to 7:00 PM ET.
References
- Centers for Disease Control and Prevention (CDC). Vaccine Storage and Handling Toolkit. Last reviewed March 29, 2024. Accessed June 25, 2024. https://www.cdc.gov/vaccines/hcp/admin/storage/toolkit/storage-handling-toolkit.pdf
- Kroger A, Bahta L, Long S, Sanchez P. General best practice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention (CDC). Updated August 1, 2023. Accessed June 25, 2024. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html
