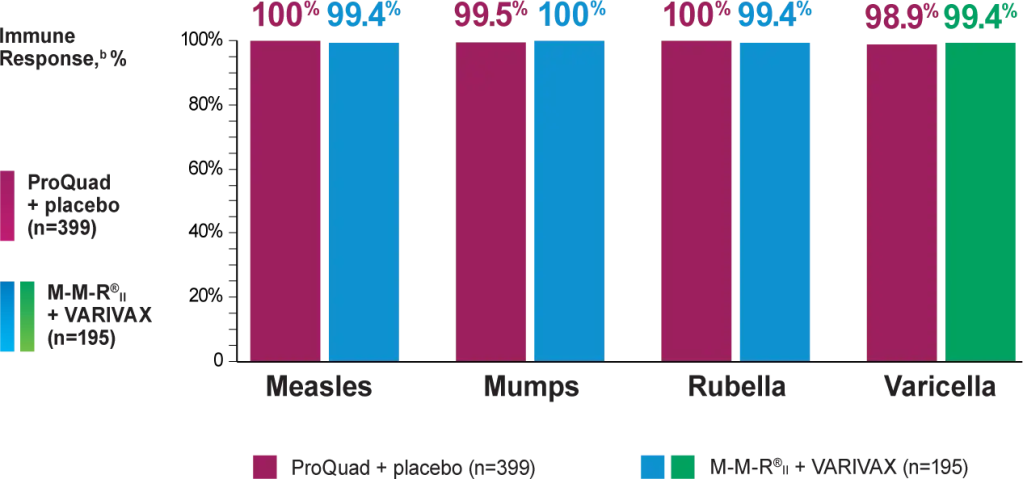
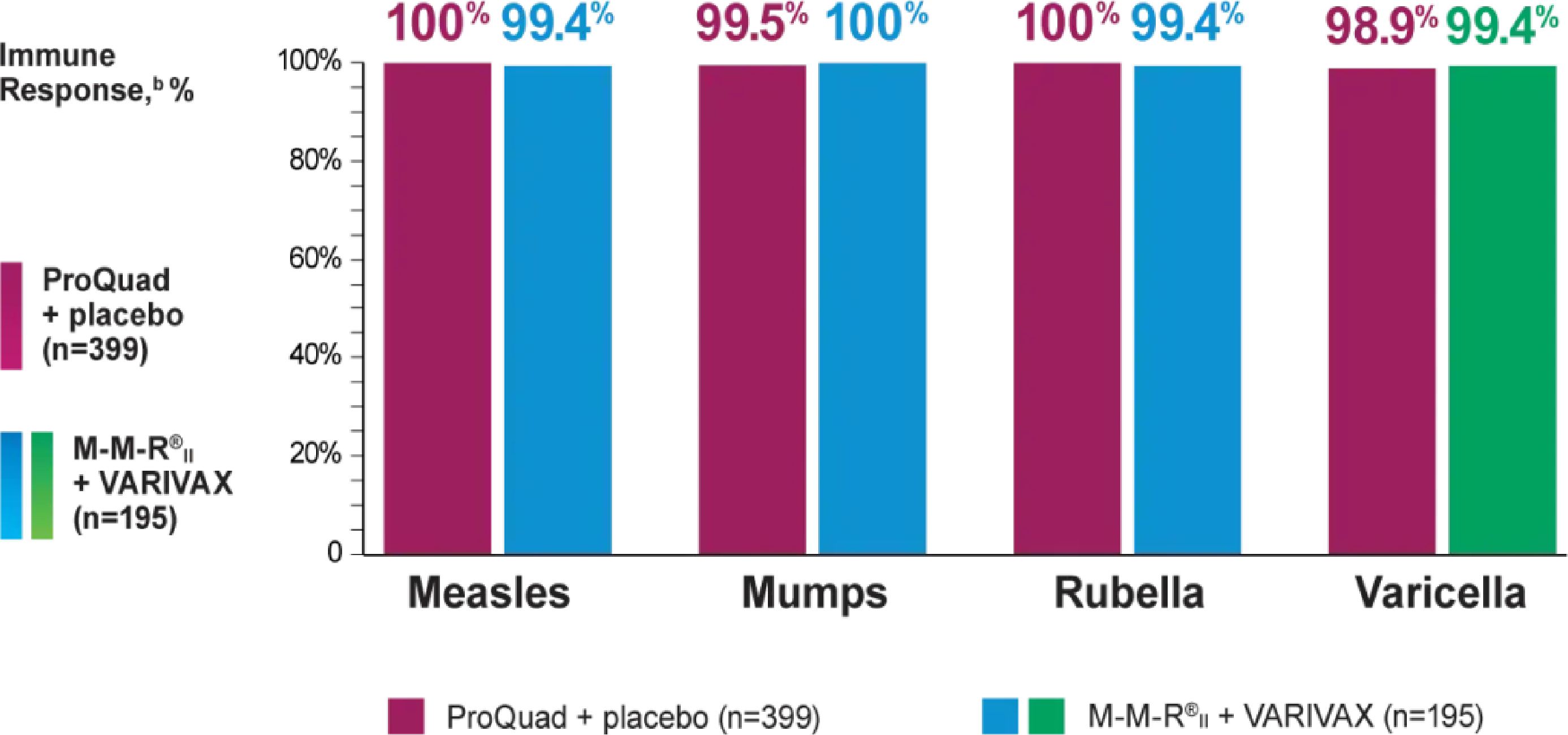
ProQuad as a 2nd MMRV vaccine dose administered subcutaneously showed a similar immune response to M-M-R®II (Measles, Mumps, and Rubella Virus Vaccine Live) and VARIVAX® (Varicella Virus Vaccine Live)
Study design
In a clinical trial, 799 healthy 4- to 6-year-old children who had received a primary vaccination with M-M-R®II and VARIVAX at least 1 month prior to study were randomized to receive ProQuad subcutaneously and placebo (n=399), M-M-R®II and placebo concomitantly at separate injection sites (n=205), or M-M-R®II and VARIVAX concomitantly at separate injection sites (n=195).
aFormal studies to evaluate the clinical efficacy of ProQuad have not been performed.
bObserved 6 weeks following vaccine dose administration.


