PedvaxHIB is contraindicated in patients with hypersensitivity to any component of the vaccine. Persons who develop symptoms suggestive of hypersensitivity after an injection should not receive further injections of the vaccine or the diluent.
As for any vaccine, adequate treatment provisions, including epinephrine, should be available for immediate use should an anaphylactoid reaction occur.
Use caution when vaccinating latex-sensitive individuals since the vial stopper contains dry natural latex rubber that may cause allergic reactions.
As with other vaccines, PedvaxHIB may not induce protective antibody levels immediately following vaccination.
There is insufficient evidence that PedvaxHIB given immediately after exposure to natural Haemophilus influenzae type b will prevent illness.
If PedvaxHIB is used in persons with malignancies or those receiving immunosuppressive therapy or who are otherwise immunocompromised, the expected immune response may not be obtained.
Special Populations
Animal reproduction studies have not been conducted with PedvaxHIB. Liquid PedvaxHIB is not recommended for use in individuals 6 years of age and older.
Geriatric use: This vaccine is NOT recommended for use in adult populations.
In clinical trials, the most frequently reported (>1%) adverse reactions, without regard to causality, were fever (≥101°F), irritability, sleepiness, injection-site pain/soreness, injection-site erythema (≤2.5 cm diameter), injection-site swelling/induration (≤2.5 cm diameter), unusual high-pitched crying, prolonged crying (>4 hours), diarrhea, vomiting, crying, pain, otitis media, rash, and upper respiratory infection.
The following additional adverse reactions have been reported with the use of the marketed vaccine: lymphadenopathy; rarely, angioedema; febrile seizures; sterile injection-site abscess.
Dosage and Administration for PedvaxHIB
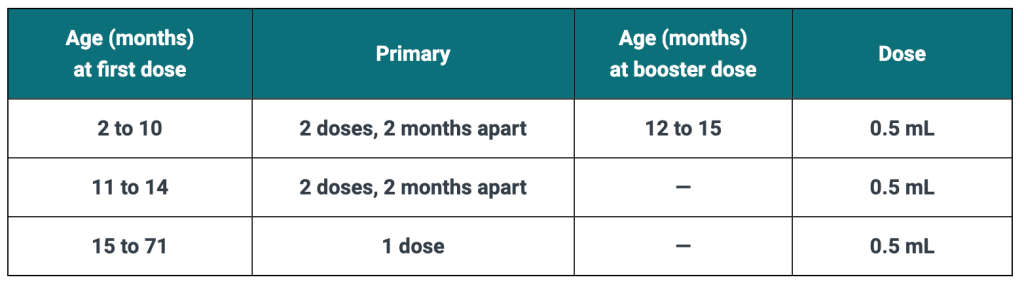
PedvaxHIB is administered in a 2-dose primary regimen before 14 months of age. Infants 2 to 14 months of age should receive a 0.5 mL dose of vaccine, ideally beginning at 2 months of age, followed by a 0.5 mL dose 2 months later (or as soon as possible thereafter). When the primary 2-dose regimen is completed before 12 months of age, a booster dose (0.5 mL) should be administered at 12 to 15 months, but not earlier than 2 months after the second dose.
Infants born prematurely, regardless of birth weight, should be vaccinated at the same chronological age and according to the same schedule and precautions as full-term infants and children.
Children 15 months of age and older previously unvaccinated against Hib disease should receive a single 0.5 mL dose of vaccine.
PedvaxHIB may be interchanged with other licensed Haemophilus b conjugate vaccines for the primary and booster doses. If PedvaxHIB is given in a series with one of the other products licensed for infants, the recommended number of doses to complete the primary series is determined by the other product and not by PedvaxHIB. PedvaxHIB may be interchanged with other licensed Haemophilus b conjugate vaccines for the booster dose.
Before administering PedvaxHIB, please read the accompanying Prescribing information.
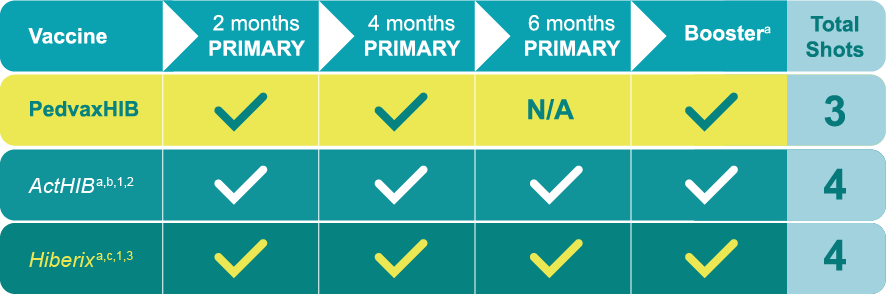
![Chart Comparing 3 Total Shots With PedvaxHIB®[Haemophilus b Conjugate Vaccine (Meningococcal Protein Conjugate)] vs. 4 Total Shots With ActHIB & Hiberix](https://www.merckvaccines.com/pedvaxhib/wp-content/uploads/sites/150/2024/03/DET_Pedvax_chart_shots_outlined.png)