Reconstitute M-M-R®II using a pre-filled diluent syringe
Attach a needle to the pre-filled syringe.
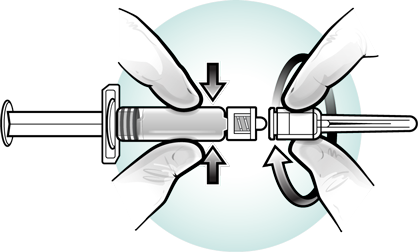

Visually inspect the vaccine for discoloration. Slowly inject the entire volume of sterile diluent prefilled syringe into the lyophilized vaccine vial. Gently agitate to dissolve completely. Discard if the lyophilized vaccine cannot be dissolved. Do not use the product if particulates are present or if it appears discolored.
Before reconstitution, the lyophilized vaccine is a light yellow compact crystalline plug. M-M-R®II, when reconstituted, is a clear yellow liquid.


Visually inspect the vaccine again for discoloration. Withdraw and administer the entire volume of the reconstituted vaccine.
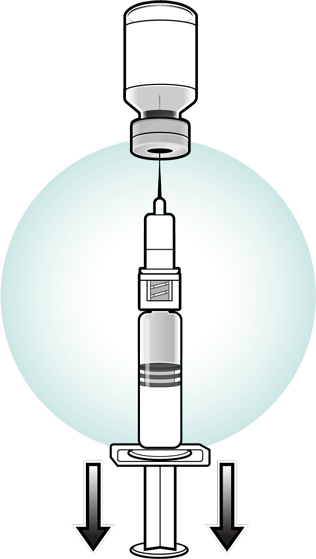

- If not used immediately, the reconstituted vaccine may be stored between 36°F to 46°F (2°C to 8°C), protected from light, for up to 8 hours. Discard reconstituted vaccine if it is not used within 8 hours.

