You have the option to choose to administer all routinely recommended injectable pediatric vaccinations included in the CDC immunization schedule via the same IM route when you choose M-M-R®II (Measles, Mumps, and Rubella Virus Vaccine Live) as your measles, mumps, and rubella vaccine.1,2
Vaccine schedule for M-M-R®II
M-M-R®II offers health care providers like you a dosing regimen to help protect children against measles, mumps, and rubella.

1st Dosea
12-15 months of age

2nd Doseb
4-6 years of age
aChildren who received an initial dose of MMR vaccine prior to their first birthday should receive additional doses of vaccine at 12–15 months of age and at 4–6 years of age to complete the vaccination series.
bSecond dose may be administered prior to 4 years of age, provided that there is a minimum interval of one month between the doses of MMR vaccine, live.
Administration of M-M-R®II
A single dose of M-M-R®II is approximately 0.5 mL. It is administered intramuscularly or subcutaneously.
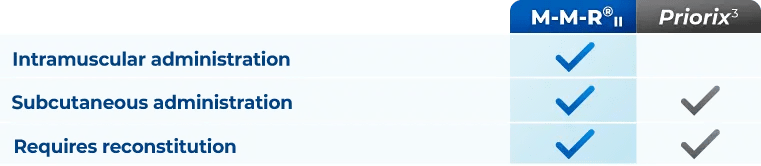
M-M-R®II continues to be latex free.
Brands mentioned are trademarks of their respective owners.
References
- Centers for Disease Control and Prevention (CDC). Administer the Vaccine(s). Last reviewed September 8, 2021. Accessed June 21, 2023. https://www.cdc.gov/vaccines/hcp/admin/administer-vaccines.html
- Wolicki J, Miller E. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 6: Vaccine administration. Centers for Disease Control and Prevention. Last reviewed April 8, 2024. Accessed October 23, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-6-vaccine-administration.html
- Priorix. Prescribing information. GlaxoSmithKline; 2024. Accessed November 1, 2024. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Priorix/pdf/PRIORIX.PDF

