Burden of disease
Pneumococcal pneumonia and IPD are a serious risk for adult patients1-4
Pneumococcal pneumonia is the most common clinical manifestation of pneumococcal disease in adults. It leads to 150,000 US hospitalizations annually and is estimated to account for 10 to 30% of all adult community-acquired pneumonia (CAP) cases in the US.5 CAP occurs when someone develops pneumonia outside of a hospital.6


Pneumococcal pneumonia leads to 150,000 hospitalizations annually in the US5
The same bacteria that cause pneumococcal pneumonia, Streptococcus pneumoniae, can invade normally sterile sites in the body, such as the blood or cerebrospinal fluid. When this occurs, it is referred to as IPD. IPD is a serious illness that can lead to hospitalization, complications including bacteremia and meningitis, and sometimes death.5
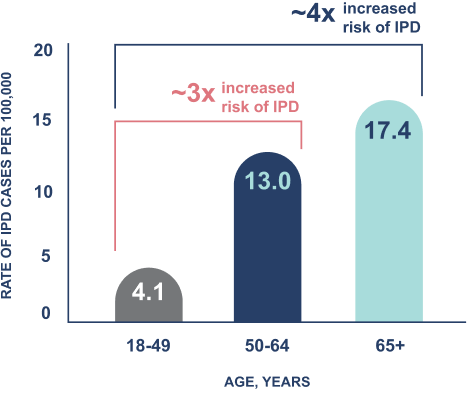
In the US, IPD is ~6x more likely to lead to death in adults over the age of 50, compared to those aged 18-49.7-12,a
Annual rate of IPD in US adults, 2018-20227-12,a

The serotypes that cause the majority of IPD cases in US adults have shifted over time1
Nearly half (~48%) of all IPD cases in adults 50+ at a national level
are caused by serotypes not included in any other PCV approved for adults1,13,14,b

These values are based on CDC epidemiologic data and do not reflect the efficacy of any pneumococcal vaccines.14
Share with health care professionals
aBased on CDC ABC surveillance data from the years 2018–2022, representing ~35 million persons and 10 states across the US. Regional variations may exist.7-12,15
bOther approved PCVs include PCV13, PCV15, and PCV20.1
ABC, Active Bacterial Core; CAP, community acquired pneumonia; CDC, Centers for Disease Control and Prevention; IPD, invasive pneumococcal disease; PCV, pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV15, 15-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; US, United States.
References:
- ABCs bact facts interactive data dashboard. SPN serotypes 1998-2022. Centers for Disease Control and Prevention. Last Updated August 28, 2024. Accessed October 16, 2024. https://www.cdc.gov/abcs/bact-facts/data-dashboard.html
- Grant LR, Meche A, McGrath L, et al. Risk of pneumococcal disease in US adults by age and risk profile. Open Forum Infect Dis. 2023;10(5):ofad192. Doi:10.1093/ofid/ofad192
- Grant LR, Meche A, McGrath L, et al. Supplementary material to: Risk of pneumococcal disease in US adults by age and risk profile. Open Forum Infect Dis. 2023;10(5):ofad192. Doi:10.1093/ofid/ofad192
- Clinical features of pneumococcal disease. Centers for Disease Control and Prevention. February 6, 2024. Accessed May 21, 2024. https://www.cdc.gov/pneumococcal/hcp/clinical-signs/
- Gierke R, Wodi P, Kobayashi M. Epidemiology and Prevention of Vaccine-Preventable Diseases (Pink Book). 14th edition. Chapter 17: Pneumococcal disease. Centers for Disease Control and Prevention. May 1, 2024. Accessed July 23, 2024. https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html
- About pneumonia. Centers for Disease Control and Prevention. October 7, 2024. Accessed October 14, 2024. https://www.cdc.gov/pneumonia/about/
- Data available on request from Merck National Service Center via email at daprequests@merck.com. Please specify information package US-PVV-00516.
- Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2018. Centers for Disease Control and Prevention. Updated May 22, 2020. Accessed June 14, 2024. https://stacks.cdc.gov/view/cdc/140450
- Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2019. Centers for Disease Control and Prevention. Updated June 16, 2021. Accessed January 14, 2025. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2019.pdf
- Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2020. Centers for Disease Control and Prevention. Updated September 20, 2022. Accessed October 15, 2024. https://stacks.cdc.gov/view/cdc/140328
- Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2021. Centers for Disease Control and Prevention. Updated June 2, 2023. Accessed January 14, 2025. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2021.pdf
- Active Bacterial Core surveillance (ABCs) report, Emerging Infections Program network, Streptococcus pneumoniae, 2022. Centers for Disease Control and Prevention. Updated July 5, 2024. Accessed September 4, 2024. https://www.cdc.gov/abcs/downloads/SPN_Surveillance_Report_2022.pdf
- Data available on request from Merck National Service Center via email at daprequests@merck.com. Please specify information package US-PVV-00504.
- 1998-2022 serotype data for invasive pneumococcal disease cases by age group from Active Bacterial Core surveillance, ages 18-49, 50-64, 65 plus, year is between 2018 and 2022. Centers for Disease Control and Prevention. Last updated July 22, 2024. Accessed August 14, 2024. https://data.cdc.gov/Public-Health-Surveillance/1998-2022-Serotype-Data-for-Invasive-Pneumococcal-/qvzb-qs6p/data
- Kobayashi M, Leidner AJ, Gierke R, et al. Expanded recommendations for use of pneumococcal conjugate vaccines among adults aged ≥50 years: recommendations of the Advisory Committee on Immunization Practices — United States, 2024. MMWR Morb Mortal Wkly Rep. 2025;74(1):1-8. doi:10.15585/mmwr.mm7401a1
